REPEAT-AF — Does adding posterior wall isolation to repeat PVI improve symptoms and outcomes?
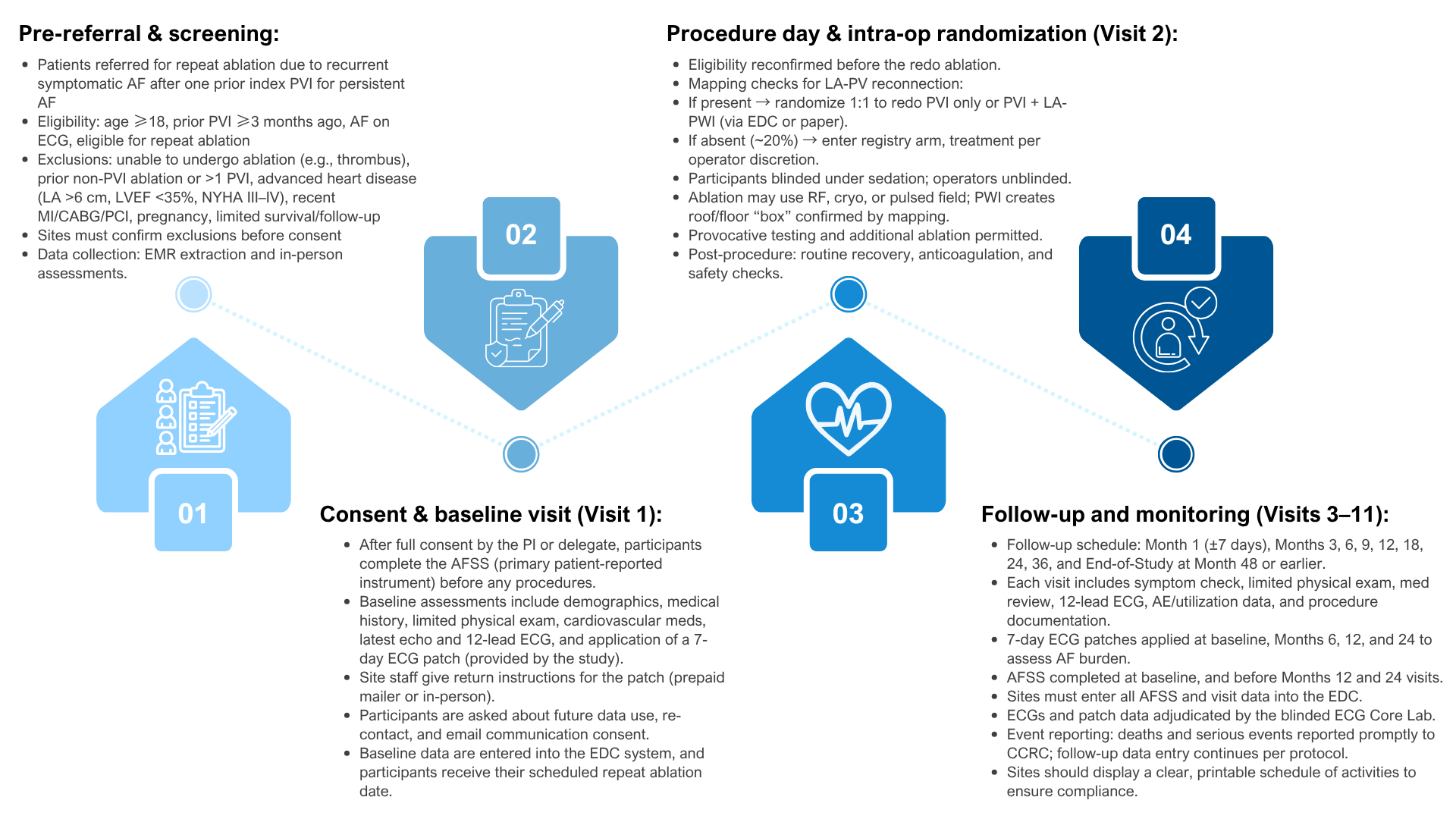
REPEAT-AF is a prospective, multicenter, single-blind randomized controlled trial designed to address an important unanswered questions in atrial fibrillation (AF) management: how to best approach patients who continue to experience symptomatic AF after a prior pulmonary vein isolation (PVI) ablation. The trial directly compares two strategies—redo PVI alone versus redo PVI plus posterior wall isolation (PWI)—to determine whether the addition of PWI improves patient outcomes. The primary outcome is the Atrial Fibrillation Severity Scale (AFSS) Symptom Score at 12 months, a validated measure of patient-reported AF burden. Secondary outcomes include time to AF recurrence, AF burden measured by ambulatory ECG monitoring, healthcare utilization, and major clinical events.
The study will enroll approximately 630 patients across 30 U.S. centers, with an initial feasibility phase followed by full-scale enrollment. Randomization occurs only if left atrial–pulmonary vein reconnection is documented during the repeat ablation procedure; patients without reconnection are enrolled into a registry arm for continued prospective follow-up. The trial is supported by the Patient-Centered Outcomes Research Institute (PCORI) and coordinated through the University of Rochester’s Clinical Cardiovascular Research Center (CCRC), with oversight from a Data Coordinating Center (DCC). These features—conditional randomization, pragmatic registry enrollment, blinded adjudication of outcomes, and multicenter collaboration—are central to ensuring results that are both rigorous and broadly applicable across diverse practice settings

Risks & Benefits
All left atrial ablation procedures involve inherent risks. The most common complications are related to vascular access, including hematomas and vascular injury at the femoral site, which are typically manageable with standard care. More serious but less frequent complications include cardiac perforation with tamponade, thromboembolic events such as stroke or TIA, atrio-esophageal fistula, phrenic nerve injury, and air or coronary embolism. For participants randomized to PWI, additional energy delivery on the posterior wall introduces theoretical increased exposure of esophageal and vagus nerve structures, requiring special vigilance. The protocol specifies safety measures including but not limited to intracardiac echocardiography, careful anticoagulation monitoring, esophageal temperature monitoring, power modulation, and phrenic nerve pacing to mitigate these risks. All adverse events are adjudicated independently, with oversight from a Data & Safety Monitoring Board to ensure ongoing participant safety.
While there is no guarantee of direct clinical benefit for any given participant, the trial is designed to capture patient-centered benefits that may result from an optimized ablation strategy. The primary anticipated benefit is reduction in AF symptom burden, measured by validated patient-reported outcomes. Secondary benefits include decreased recurrence of atrial arrhythmia, reduced AF burden on ambulatory monitoring, fewer hospitalizations and emergency visits, and reduced serious clinical events. Patients also receive logistical support such as study-provided 7-day ECG monitoring patches and compensation of $50 for each completed non-procedure study visit. Even if an individual does not experience symptomatic improvement, their participation advances the field by generating much-needed evidence to guide future practice in persistent AF
Risks
Benefits
Participation journey
Resources
